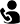iLAMP Novel-CoV19 Detection Kit
Product Details:
iLAMP Novel-CoV19 Detection Kit Price And Quantity
- 1 Box
iLAMP Novel-CoV19 Detection Kit Trade Information
- Yes
Product Description

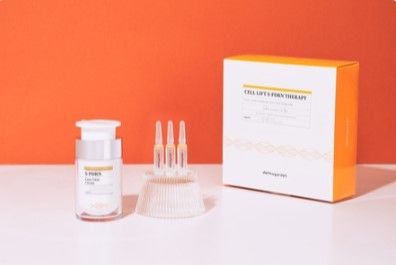
Product Feature
$5.00/1 test
Min.Order : 100 tests
1 box(kit) = 2000 tests
High speed : 20 minutes
High Accuracy : Close to 100%
Low Shipping Cost : 2000 tests in 1 box
Easy Preparation : 3 tubes only
High Throughput : about 2,500 tests per day
Our In-vitro test kit enables precise and efficient detection of the Coronavirus(2019-nCoV). Our testing kit is based on Real-time RT LAMP PCR (Real-time Reverse Transcription Loop-mediated Isothermal Amplification PCR) method and is intended for use with upper respiratory specimen collected from nasopharyngeal and oropharyngeal swabs. The iLAMP Novel-CoV19 assay's key differentiator from current rRT PCR COVID19 assays is the ability to test in 20 minutes by amplifying under one temperature condition without heat-induced denaturation.
Our company

iONEBIO has been having an indisputable achievement and growth in molecular biology, virus and disease diagnosis sectors.
Based on the company's proficiency and expertise, we have introduced the 25min COVID-19 LAMP test kit and exported the FICS(One-stop 1 hour COVID-19 test platform) at the airport of Paraguay and recently developed the CoV19 Neutralizing Antibody Kit.
Rapid and Reliable COVID-19 Detection
The iLAMP Novel-CoV19 Detection Kit provides healthcare professionals with a swift and dependable method to identify SARS-CoV-2 infections. By harnessing isothermal amplification, this kit streamlines viral detection without the need for sophisticated laboratory equipment, ensuring results in less than an hour. Its high sensitivity enables detection at low viral loads, making it a practical solution for timely diagnosis and patient triage.
User-friendly Visual Readout
Unlike conventional PCR assays that demand advanced instruments, the iLAMP kit utilizes a colorimetric readout. Results are interpreted visually, minimizing user error and reducing operational complexities. This design makes the kit accessible in various healthcare settings, from well-equipped labs to point-of-care environments, facilitating broader-scale testing with minimal training.
Comprehensive and Safe Package
Each kit contains all essential components, including reaction tubes, reagent solutions, and positive and negative controls, along with a clear user manual. It is validated as per ICMR guidelines, assuring regulatory compliance and reliable performance. The compact format (25 tests/kit) and reasonable shelf life optimize storage and transportation, supporting distributors, exporters, and healthcare facilities alike.
FAQ's of iLAMP Novel-CoV19 Detection Kit:
Q: How does the iLAMP Novel-CoV19 Detection Kit work?
A: The kit operates on the Loop-Mediated Isothermal Amplification (LAMP) method. It detects SARS-CoV-2 genetic material in throat or nasal swab samples and produces a visual color change indicating positive or negative results. No advanced laboratory devices are required, making the process straightforward and user-friendly.Q: What are the main benefits of using this detection kit over traditional PCR?
A: Unlike PCR, the iLAMP kit eliminates the need for complex thermal cyclers. It delivers highly sensitive and specific results in less than one hour through a simple color change, making it ideal for quick and large-scale screening in clinical or field settings.Q: Who is the intended user for the iLAMP Novel-CoV19 Detection Kit?
A: This kit is intended for professional use only, including medical laboratory staff and healthcare professionals. It is not suitable for direct use by untrained individuals due to the need for correct sample collection and interpretation.Q: Where and how should the kit be stored to ensure optimal performance?
A: To maintain reagent integrity and accuracy, the kit should be stored between 2C and 8C. Proper storage conditions help preserve its 12-month shelf life and ensure reliable test results throughout its use.Q: What samples can be tested with this kit and what is the detection limit?
A: The kit is validated for use with throat and nasal swab samples. It can detect as few as 10 copies of viral RNA per reaction, reflecting its high analytical sensitivity and reliability in early or low-level infections.Q: Is there guidance available for using the kit, and what materials are included?
A: A detailed user manual is included with each kit, guiding users through sample preparation, testing workflow, and result interpretation. Each kit contains reaction tubes, reagent solutions, and both positive and negative controls for comprehensive quality assurance.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese